Revision:Why is benzene stable?
第1页/共1页
 Revision:Why is benzene stable?
Revision:Why is benzene stable?
* The p orbitals on each carbon atom in benzene overlap
* There is delocalisation of electrons in the ring (not 3 localised C==C double bonds)
* This makes benzene more thermodynamically stable than predicted
* It therefore undergoes substitution reactions rather than
addition reactions because addition reaction break the delocalisation
in the ring. e.g The nitration of benzene results in a loss of H+ which
is replaced by NO2+
Diagram of Benzene
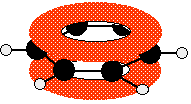
* There is delocalisation of electrons in the ring (not 3 localised C==C double bonds)
* This makes benzene more thermodynamically stable than predicted
* It therefore undergoes substitution reactions rather than
addition reactions because addition reaction break the delocalisation
in the ring. e.g The nitration of benzene results in a loss of H+ which
is replaced by NO2+
Diagram of Benzene

第1页/共1页
您在这个论坛的权限:
您不能在这个论坛回复主题
 首页
首页
